MORE FROM THE STACKS
EDITOR’S NOTE: There are literally thousands of journals published around the world that relate to the disability community. It is virtually impossible to capture even a fraction of them. HELEN receives "stacks" of journals and selectively earmarks what we feel are "must read" articles of interest for our readers. It's a HELEN perk
National Disability Employment Awareness Month
October 2025 marks the 80th anniversary of National Disability Employment Awareness Month, an annual milestone moment to reflect on inclusion in the workplace. In 2024, the U.S. Bureau of Labor Statistics reported that the employment-population ratio – the proportion of the population that is employed – was 22.7% among those with a disability.
Currently, unemployment among people with autism is approximately 85%, and at least 500,000 more individuals with autism will enter the workforce in the next decade. Individuals with autism are often unable to navigate the social dynamics and can face substantial challenges navigating the workplace.
The D’Eri family founded Rising Tide Car Wash, a pioneering social enterprise with a primary mission to employ adults with autism and the only multi-unit, family-owned car wash in South Florida. Their mission-driven business has turned a staggering reality into an inspiring success story.
Rising Tide Car Wash is one of the largest employers of people with autism in the U.S., with approximately 90 employees with autism and three locations across South Florida – Parkland, Margate and Coral Springs. Rising Tide Car Wash continues to expand and was recently approved to open a fourth South Florida location in Pompano Beach in Q4 2026 or Q1 2027.
Thomas D’Eri and his father, John D’Eri, co-founded this innovative social enterprise to develop a scalable solution for employing individuals with autism in 2013. They were inspired to do this by watching John’s son and Thomas’ brother Andrew, a vibrant young man with autism, struggle to find his place in the world. Rising Tide Car Wash has created an innovative model that intentionally designs the work environment in a way that empowers individuals with autism to thrive.
The D’Eris’ purpose is to help people shine. They strive to change the root cause of this unemployment: society views autism as a disability, as opposed to diversity. Rising Tide Car Wash boasts an alumni network of over 100 individuals with autism who have moved on to new jobs or pursued higher education, proving the value that people with autism bring to the workforce.
During National Disability Employment Awareness Month, Thomas D’Eri participated as a keynote speaker at the Els for Autism 2025 Employer-to-Employer Summit. The summit brought employers together to learn about the benefits of employing individuals with autism spectrum disorder and developing a workplace culture that promotes inclusion of all. D’Eri spoke to guests about Rising Tide’s sales program and how designing systems for neurodivergent team members leads to better outcomes for everyone.
Thomas D’Eri is also the author of the best-selling book, “The Power of Potential,” published by Harper Collins Leadership, a book that highlights employment opportunities for individuals with autism and addresses common challenges businesses face.
—
Rising Cognitive Disability as a Public Health Concern Among US Adults
Trends From the Behavioral Risk Factor Surveillance System, 2013–2023
(10/21/2025 Issue; Open Access)
Ka-Ho Wong Christopher D. Anderson , Cecilia Peterson, Erin Bouldin, Lauren Littig Neeharika Krothapalli, Trieste Francis Yvonne Kim, Giselle Cucufate, Kevin Navin Sheth Adam de Havenon
Abstract
Background and Objectives
Cognitive disability—defined by the Behavioral Risk Factor Surveillance System (BRFSS) as serious difficulty concentrating, remembering, or making decisions because of a physical, mental, or emotional condition—has become the most commonly reported disability among US adults. This broad definition reflects a heterogeneous range of underlying causes and highlights the growing public health significance of cognitive disability in the population. Previous studies have identified disparities by race, age, and socioeconomic status, but few have examined how these patterns have evolved over the past decade. This study analyzes national trends in self-reported cognitive disability from 2013 to 2023 using BRFSS data, with a focus on differences across age groups, racial and ethnic populations, and key social determinants of health.
Methods
We conducted a retrospective analysis using data from the Centers for Disease Control and Prevention's Disability and Health Data System, which integrates nationally representative responses from US adults (aged ≥18 years) in the BRFSS from 2013 to 2023, excluding 2020 and participants who self-reported depression, to better identify nonpsychiatric cognitive impairment. The primary outcome was self-reported cognitive disability, defined as “serious difficulty concentrating, remembering, or making decisions.” Survey-weighted logistic regression was used to model prevalence trends and examine associations with demographic and socioeconomic factors.
Results
From 2013 to 2023, a total of 4,507,061 responses were included in the analysis. Apart from analyses focusing on strata of age, all estimates of cognitive disability were age-adjusted. Most respondents were aged 18–39 years (36.8%), identified as non-Hispanic White (60.9%), and had completed at least a high school education (87.3%). The age-adjusted self-reported cognitive disability prevalence in the United States rose from 5.3% (95% CI 5.1%–5.4%) in 2013 to 7.4% (95% CI 7.2%–7.6%) in 2023, with statistically significant increases beginning in 2016. The prevalence of cognitive disability among younger adults aged 18–39 years nearly doubled, increasing from 5.1% (95% CI 4.8%–5.3%) to 9.7% (95% CI 9.2%–10.2%), making this age group the primary driver of the overall rise in cognitive disability in the United States.
Discussion
The disproportionate growth in cognitive disability among younger adults seems to be the primary driver of the overall national trend. These findings warrant further investigation, given their potential long-term implications for population health, workforce productivity, and health care systems.
Introduction
Disability affects more than 70 million adults in the United States, representing over 1 in 4 individuals. A 2022 analysis from the Behavioral Risk Factor Surveillance System (BRFSS) reported this prevalence, highlighting a significant increase compared with previous estimates.1 This surpasses figures reported in the 2016 BRFSS analysis conducted by the Centers for Disease Control and Prevention (CDC), which estimated that approximately 61 million US adults—around 1 in 4—were living with a disability.2 The increase in disability prevalence from 2016 to 2022 is likely attributable in part to the long-term effects of coronavirus disease 2019 (COVID-19). This is supported by data showing that the prevalence of long COVID symptoms was higher among individuals with disabilities (10.8%) compared with those without disabilities (6.6%).1 Furthermore, the 2016 BRFSS analysis identified cognitive disability as the second most common type of disability in the United States, with a prevalence ranging from 6% to 11%.2-4 The 2022 BRFSS report, however, indicated that cognitive disability had become the most frequently reported disability, with a prevalence of 14%.5
While robust literature has demonstrated that subjective self-reported cognitive impairment is associated with greater risk of future cognitive decline in older adults (≥60 years),6 it remains unclear whether similar associations exist in younger populations. Previous trend analysis studies examining self-reported cognitive impairment between 1997 and 2015 have observed disparities in the prevalence of cognitive disability, with higher rates observed among Black, American Indian/Alaska Native (AI/AN), and Hispanic populations.7 These disparities also vary based on age, nativity, and socioeconomic factors.3,4,8-10 In addition, adults with cognitive disabilities are less likely to receive preventive health screenings and are more likely to have chronic conditions compared with individuals without disabilities.8 However, previous analyses have not evaluated the impact of cognitive disability in cohorts excluding individuals with depression, which may confound associations with cognitive impairment.10 Existing literature demonstrates that depression can exacerbate both subjective and objective cognitive symptoms, with evidence supporting a bidirectional relationship between depressive symptoms and cognitive function over time.11-15 In addition, people younger than 60 years have not been specifically examined in these long-term studies, limiting our understanding of whether the prevalence of cognitive disability has changed over time in younger adults.
To determine whether associations between race, age, and other social determinants of health factors persist over a longitudinal period, we used data from the CDC Disability and Health Data System (DHDS), derived from the BRFSS from 2013 to 2023.16
Our analysis extends beyond previous research to incorporate a spectrum of demographic and socioeconomic factors, providing a more comprehensive understanding of the evolving cognitive disability landscape. The findings from this study are intended to inform future research initiatives aimed at addressing the increasing burden of cognitive disability.
—
Investment in Alzheimer's Disease Research for the Next Generation of Adults with Down Syndrome Will Yield Health Benefits for Future Generations
(First published: 09/10/2025 – Open Access - https://doi.org/10.1002/alz.70348)
Margaret M. Weden, Lori Frank, Andrew W. Dick, Zetianyu Wang, Susan Peschin, Diane E. Bovenkamp, Sharyn L. Rossi, Dana Sciullo, Hampus Hillerstrom, Richard A. Fisher
ABSTRACT
Recent innovations in Alzheimer's disease (AD) treatment highlight critical gaps in knowledge about how to support healthy aging of adults with Down syndrome (DS). RAND researchers updated demographic and epidemiological evidence about the DS population to assess the impact of increased investment in treatment innovations for DS-associated Alzheimer's disease (DS-AD). They estimated life expectancy at birth in 2020 to be 55 years, with ≈ 5 years of DS-AD. They found that the results of investment were dramatic. Between 2020 and 2070, adult years of life are expected to increase by 5 years without any increase in unhealthy years of life with DS-AD. Caregiving hours for individuals with DS-AD are expected to be reduced by 40%, which will generate large annual savings. The new evidence underscores the magnitude of the impact that investment in DS-AD treatments could have for individuals with DS, their families, and caregivers.
Highlights
Evidence is sparse about treatment for Down syndrome (DS)-associated Alzheimer's disease (DS-AD) and healthy aging of DS adults.
This population simulation model estimates DS-AD caregiving costs at ≈ $1 billion per year.
DS-AD innovations could increase life expectancy by 5 years and reduce caregiving by 40% by 2070.
This better forecasting can improve policy and service planning.
DS-AD research investment could yield dramatic gains for individuals and families.
We live in an exciting age for Alzheimer's disease (AD) research. Since 2021, the US Food and Drug Administration (FDA) has now approved two disease-modifying treatments for the disease,1 representing the first major breakthroughs in many years. Additional new treatments are in the pipeline.2 Yet, people with intellectual and developmental disabilities are underrepresented, and often excluded, from clinical trials.3, 4
Disparities in access are particularly striking for individuals with Down syndrome (DS). Despite the key role that individuals with DS have played in the basic science, discovery, and development phases of new treatment development, individuals with DS have typically been excluded in the clinical research, efficacy, and safety phases of development. With respect to the most recently approved treatment innovations for AD, there has not yet been any testing for safety or efficacy in people with DS, and at this time, DS experts do not recommend the widespread use of these treatments in adults with DS until clinical trials for safety take place.5 The first safety trial with the drug Kisunla was announced at an Eli Lilly session at AAIC 2024 and is expected to start enrolling in 2025.
The unequal inclusion of individuals with DS in all phases of the treatment development process has likely arisen from the strict ethical requirements for the protection of risks of harm and heightened scrutiny of risks for a vulnerable population. Thankfully, through advocacy efforts over the last 2 years, great progress has taken place including: confirmation from the US Centers for Medicare and Medicaid (CMS) that people with DS are covered for the newly approved anti-amyloid antibody drugs; confirmation from the FDA that people with DS are included in the label of these drugs; and the recommendations from a group of 25 experts that defined accommodations to prior authorization criteria to access these drugs across the US states.6 However, it remains the case that the lack of evidence about the potential impact of AD treatment innovation for the individuals with DS and their families operates to widen disparities in the consequences of AD for the DS population relative to the general population that magnifies and exacerbate the uniquely great predisposition for AD faced by individuals with DS.
The possibility that new treatment innovations might be able to slow the dramatic increases in AD expected in the twenty-first century offers an exciting solution to a complex public health and health services planning problem shown to be driven by demographic change in the twentieth century.7 Research has shown that the aging of the Baby Boom cohort of adults born between 1946 and 1964 has generated large increases in the number of US adults with AD in the total US population, and that balance of childbearing and survival trends for birth cohorts born after the Baby Boom will further expand the size of the AD population.8, 9 In addition, however, research has also shown that advances in therapeutic and preventive strategies that result in modest delays in the onset and progression of AD can significantly reduce the burden of the disease.10, 11 These are exciting findings even in the context of current and expected treatments in the pipeline.
Unfortunately, and in contrast with the above noted rapid expansion of population forecasting evidence supporting health services planning for AD in the general population, there is scant evidence about the demographics and health of the adult population with DS. The gaps in the evidence about aging with DS raise the following questions: What are the current and future trends in the demographic and epidemiological characteristics of the adult population with DS? What are the trends in DS-associated Alzheimer's disease (DS-AD) in the United States? How might research investment affect trends in cognitive health, longevity, and caregiving in the next 50 years? Answers to these questions are needed so we know what to research and where to invest.
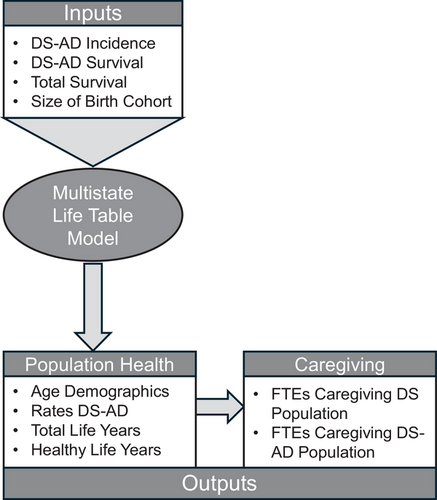
To begin to address these questions, RAND researchers recently developed a population simulation and projection model to predict how many babies born with DS will grow to adulthood and how many will develop AD. A simplified depiction of the RAND simulation and projection model for DS-AD is provided in Figure 1, and the methodological details are described elsewhere in the publicly accessible RAND Report.12 The RAND researchers’ approach was to adapt a well-established demographic method for population simulation of current trends and projection of future trends (i.e., the multistate life table [MSLT]) to the available data on aging and health of the DS population. The MSLT provided the structure for defining and abstracting parameters for a model that could be informed, respectively, from the best available epidemiological evidence (i.e., about the incidence of DS-AD and the likelihood of survival with DS-AD) and demographic evidence (i.e., about the changing size and survival patterns of DS birth cohorts). The MSLT was especially advantageous because it provided a simple and flexible structure for the RAND researchers to abstract and harmonize findings across studies, addressing gaps and differences across studies in reporting of the findings.
Figure 1
Framework for multistate population simulation and projection of Down syndrome–associated Alzheimer's disease. The figure is adapted from documentation and visualizations of the data and methods from a recent report by the RAND Corporation.12
The MSLT was refined through an iterative process of parameterization and calibration that entailed: (1) probabilistic selection of an initial set of age-specific input parameters that fell within the boundaries of prior literature, (2) calculation of outputs from inputs using MSLT equations, (3) evaluation of the MSLT output respective to calibration targets from the prior literature (i.e., most notably a recent meta-analysis,13 and, as appropriate, (4) re-estimation with a new probabilistic draw of age-specific input parameters. The range of age-specific input parameters for the MSLT was defined using both primary data estimates and secondary data estimates. The primary data estimates were made by the project team using demographic and epidemiological surveillance data from the National Center for Health Statistics and the CMS. The secondary data estimates are from studies that either used similar subsets of these epidemiological and demographic surveillance data,14, 15 or studies that used large clinical panels.16, 17
The RAND study first used their model for population simulation of the current size and characteristics of the population of adults with DS. This updated demographic evidence on the DS population from an earlier study, which the RAND research team obtained from the authors.14 The RAND model estimated that between 2000 and 2020, there was a large increase in the percentage of the total DS population who reached older adulthood (defined here as age ≥ 50 years). Improvements in survival captured in the model were partially responsible for this increase in the older adult DS population, which is also referred to as population aging. In addition, changes in the number of individuals born each year with DS were also a critical factor. By ≈ 2000, the individuals who entered older adulthood came from the Baby Boom cohort, which was relatively larger in size at birth than birth cohorts before or after. As a consequence of the increasingly larger size of an increasingly older population of adults with DS, the RAND study predicted that the prevalence of DS-AD increased dramatically between 2000 and 2020: from ≈ 17% in 1990 and 2000 to > 20% in 2010 and nearly 30% in 2020.
The RAND study next used their model for population projection. The model was used to forecast the health and longevity of individuals with AD 50 years in the future, imagining that innovations achieve the “modest” goals projected in studies on the general population, that is, that the onset of AD is delayed by ≈ 1% per year.7, 10, 11 Critically, however, the RAND study imagined a future in which these innovations become widely available to people with DS.
As explained in detail in the RAND Report, the assumptions for the expected change in incidence inputs over the next 50 years are informed by prior research and tailored to the DS population in consultation with the stakeholders and key informants. For changes in survival, the RAND study considered several options, but we present their most optimistic scenario in which age-specific mortality for adults with DS was projected to decrease by ≈ 0.7% every year over the next 50 years, a rate similar to the decline seen in the general population over the twentieth century.18 Under these conditions, the RAND study estimated that total life expectancy at birth for individuals born with DS increased from ≈ 55 years in 2020 to ≈ 58 in 2070.
For changes in the incidence of DS-AD, the RAND study also considered several options; we present here the most optimistic, with an accumulative annual decrease in age-specific DS-AD incidence of 1% per year. The resulting delay in the age of onset of DS-AD is described in recent projections of AD in the general population as “modest,” and the twentieth-century improvements in treatment for congestive heart failure and Parkinson's disease are described in these studies as the prior historical precedence for this magnitude of change in disease incidence being achieved through treatment innovation.10, 11 First, the RAND model findings shown in Figure 2 estimate the status quo in 2020: adults will spend on average ≈ 12 years of life after age 45 without DS-AD and 5 years with DS-AD, so that the expected age of DS-AD onset is 57 years and total life expectancy after age 45 is 62 years. Moreover, the RAND model projects that—under the above noted conditions, in which the expected improvements in AD for the general population are extended to the DS population for DS-AD—total life expectancy among adults surviving to age 45 in 2070 will reach 67 years, with all of the change occurring in the number of life-years without DS-AD. In other words, among adults with DS surviving to age 45 in 2070, RAND projects that the age of onset of DS-AD and total life expectancy will increase equally by ≈ 5 years, so that the gains in longevity extend the years of life prior to the onset of the disease.
Figure 2
Predicted life expectancy for US adults with Down syndrome, by DS-AD status, in 2020 and 2070. Predicted life expectancy is estimated using data and methods documented in a recent report by the RAND Corporation.12 DS-AD, Down syndrome–associated Alzheimer's disease.
Finally, the RAND study used prior evidence about the differences in caregiving needs of adults with DS, depending on whether they have developed DS-AD, to project the impact of the innovations in AD treatment on caregiving. On the basis of a review of the literature and discussions with key informants, the RAND researchers identified inputs for the expected caregiving hours per day for an individual with and without DS-AD; that is, caregiving hours for individuals with DS-AD were estimated at 8.3 hours per day (or 3029.5 hours per year) versus 2.5 hours per day (or 912.5 hours per year) for individuals without DS-AD. Although these rates came from a specific, large study,19 other studies reported similar estimates in the range of 8 to 10 hours for DS-AD and equally ≈ 2.5 hours for no DS-AD.20-22 The RAND model predicted that if the above-noted scenario becomes a reality (i.e., the life years of individuals with DS are increased by 5 years without increasing the number of unhealthy years with DS-AD), there would be a substantial decrease in the total caregiving needs of the adult DS population compared to the total caregiving needs for the population in the absence of these innovations.
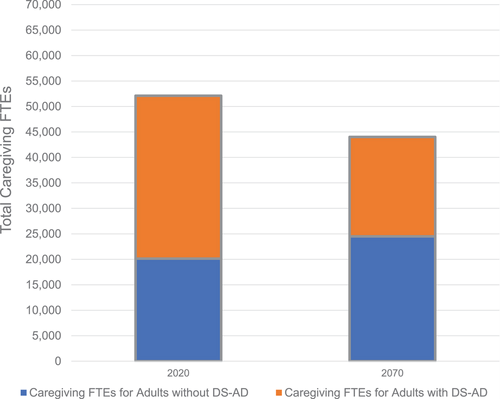
As shown in Figure 3, the RAND study determined that in 2020, based on the number of adults with and without DS-AD predicted using the model, care for adults with DS would have required ≈ 52,000 full-time equivalent (FTE) hours of caregiving (which is equivalent to 52,000 caregivers working 40 hours per week providing care to individuals with DS). This total included ≈ 32,000 FTE hours of caregiving for adults with DS-AD. When hourly rates and lost wages of those who forgo work to care for a loved one are considered, the economic cost of caregiving of adults with DS-AD is ≈ $1 billion. If, over the next half-century, treatment innovations reduce the incidence of DS-AD as hoped, the amount of caregiving time needed for adults with DS-AD would fall by ≈ 12,500 FTE hours, or by nearly 40% from 2020 levels.
Figure 3
Predicted full-time equivalent hours (FTEs) for caregiving of individuals with Down syndrome ages ≥ 45 years. Predicted FTEs are estimated using data and methods documented in a recent report by the RAND Corporation.12 DS-AD, Down syndrome–associated Alzheimer's disease.
Several shifts in policy—at government agencies, insurers, drug developers, hospitals, and doctors’ offices—would help make sure that people with DS get their best shot at a long and healthy life.
First, clinical trials of AD treatments must include individuals with DS. The exclusion of this population is not only a failure of health equity, but it is also a lost opportunity. Adults with DS have a very high unmet medical need for treatment and services. In addition, they offer a valuable resource to the entire AD community for the development of future cross-indication and real-world treatments, as well as the opportunity to better understand and distinguish the pathophysiology of the disease from the normal effects of aging.
Second, the FDA should work with industry drug developers and research-focused DS non-profit organizations to establish feasible study size endpoints. This should be conducted under a clear regulatory path and with attention to the limited number of research participants in this population.
Third, doctors and other providers need training in how to properly prescribe and monitor AD drugs among patients with DS-AD.
Finally, health-care providers and caregivers who deal with adults with DS should be educated on how to identify, manage, and treat DS-AD. Any initiatives to improve early detection of AD should include people with DS. At the moment, the US health-care system is not equipped to address the increase in DS-AD. However, with more investment and research, along with policy changes, we can greatly reduce future expenditures. Most importantly, we can improve the lives of adults with DS as they grow older.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the key informants, stakeholders, and independent reviewers of the RAND Corporation research study described herein. Special thanks are extended to Gert de Graaf for providing us with demographic data projections for the DS population. This research study was supported by the LuMind IDSC Foundation, Alliance for Aging Research, BrightFocus® Foundation, and National Down Syndrome Society.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
The study was approved by the RAND Human Subjects Protections Committee (2022-N0601). In addition, the lead authors (Weden, Frank, Dick, and Wang) have signed and are held accountable to the RAND Corporation Institutional Principles, Code of Ethical Conduct, and conflict of interest policies which require independence and objectivity of research activities and which protect against both real and perceived conflicts of interest regarding research support or financial involvement.
Supporting Information
REFERENCES
1 US Food and Drug Administration. FDA news release: FDA converts novel Alzheimer's disease treatment to traditional approval. 2023; https://www.fda.gov/news-events/press-announcements/fda-converts-novel-alzheimers-disease-treatment-traditional-approval
2 Teng Z. Novel development and prospects in pathogenesis, diagnosis, and therapy of Alzheimer's disease. J Alzheimers Dis Rep. 2024; 8(1): 345-354.
PubMed Web of Science® Google Scholar
3Hillerstrom H, Simons JS. People with Down syndrome deserve access to Alzheimer's treatment. The Boston Globe. 2018.
4 Shariq S, Cardoso Pinto AM, Budhathoki SS, Miller M, Cro S. Barriers and facilitators to the recruitment of disabled people to clinical trials: a scoping review. Trials. 2023; 24(1): 171.
PubMed Web of Science® Google Scholar
5Hillerstrom H, Fisher R, Janicki MP, et al. Adapting prescribing criteria for amyloid-targeted antibodies for adults with Down syndrome. Alzheimers Dement. 2024; 20(5): 3649-3656.
CAS PubMed Web of Science® Google Scholar
6Hillerstrom H, Fisher R, Janicki MP, The Working Group on Criteria for Access to Alzheimer's Therapeutics for Adults with Down Syndrome. Adapting eligibility criteria for prescribing FDA approved anti-amyloid immunotherapeutics for adults with Down syndrome with early stage Alzheimer's dementia. Lumind IDSC and the National Task Group. 2023. https://lumindidsc.org/wp-content/uploads/2023/06/Working-Group-DS-AD-Eligibility-Criteria-May-30-2023.pdf
7 Collaborators GBDDF. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022; 7(2): e105-e125.
PubMed Web of Science® Google Scholar
8 Rutter CM, Edochie I, Friedman EM, Slaughter ME, Weden MM. A simple method for simulating dementia onset and death within an existing demographic model. Med Decis Making. 2022; 42(1): 43-50.
PubMed Web of Science® Google Scholar
9 Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer's disease and mild cognitive impairment in the United States (2020-2060). Alzheimers Dement. 2021; 17(12): 1966-1975.
PubMed Web of Science® Google Scholar
10 Sloane PD, Zimmerman S, Suchindran C, et al. The public health impact of Alzheimer's disease, 2000-2050: potential implication of treatment advances. Annu Rev Public Health. 2002; 23: 213-231.
PubMed Web of Science® Google Scholar
11Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998; 88(9): 1337-1342.
CAS PubMed Web of Science® Google Scholar
12Weden MM, Wang Z, Frank L, Dick AW, Marsolais E. Modeling the Impact of Research Investment on Down Syndrome – Associated Alzheimer's Disease (RRA2663). RAND Corporation; 2023.
13Iulita MF, Garzon Chavez D, Klitgaard Christensen M, et al. Association of Alzheimer disease with life expectancy in people with Down syndrome. JAMA Netw Open. 2022; 5(5):e2212910.
PubMed Web of Science® Google Scholar
14 de Graaf G, Buckley F, Skotko BG. Estimation of the number of people with Down syndrome in the United States. Genet Med. 2017; 19(4): 439-447.
PubMed Web of Science® Google Scholar
15Rubenstein E, Hartley S, Bishop L. Epidemiology of dementia and Alzheimer disease in individuals with Down syndrome. JAMA Neurol. 2020; 77(2): 262-264.
PubMed Web of Science® Google Scholar
16 Lai F, Mhatre PG, Yang Y, Wang MC, Schupf N, Rosas HD. Sex differences in risk of Alzheimer's disease in adults with Down syndrome. Alzheimers Dement (Amst). 2020; 12(1):e12084.
17 Mhatre PG, Lee JH, Pang D, et al. The Association between sex and risk of Alzheimer's disease in adults with Down syndrome. J Clin Med. 2021; 10(13): 2966.
CAS PubMed Web of Science® Google Scholar
18 Bell FC, Miller ML. Life tables for the United States Social Security Area, 1900-2100. Social Security Administration, Office of the Chief Actuary, 2005.
19 Janicki MP, Dalton AJ, McCallion P, et al. Group home care for adults with intellectual disabilities and Alzheimer's disease. Dementia. 2005; 4.
20 Cleary J, Doody O. Nurses' experience of caring for people with intellectual disability and dementia. J Clin Nurs. 2017; 26(5-6): 620-631.
PubMed Web of Science® Google Scholar
21 Courtenay K, Jokinen NS, Strydom A. Caregiving and adults with intellectual disabilities affected by dementia. J Policy Pract Intel. 2010; 7(1): 26-33.
Web of Science® Google Scholar
22 McCarron M, McCallion P, Reilly E, Mulryan N. A prospective 14-year longitudinal follow-up of dementia in persons with Down syndrome. J Intell Disabil Res. 2014; 58(1): 61-70.
CAS PubMed Web of Science® Google Scho